[ad_1]
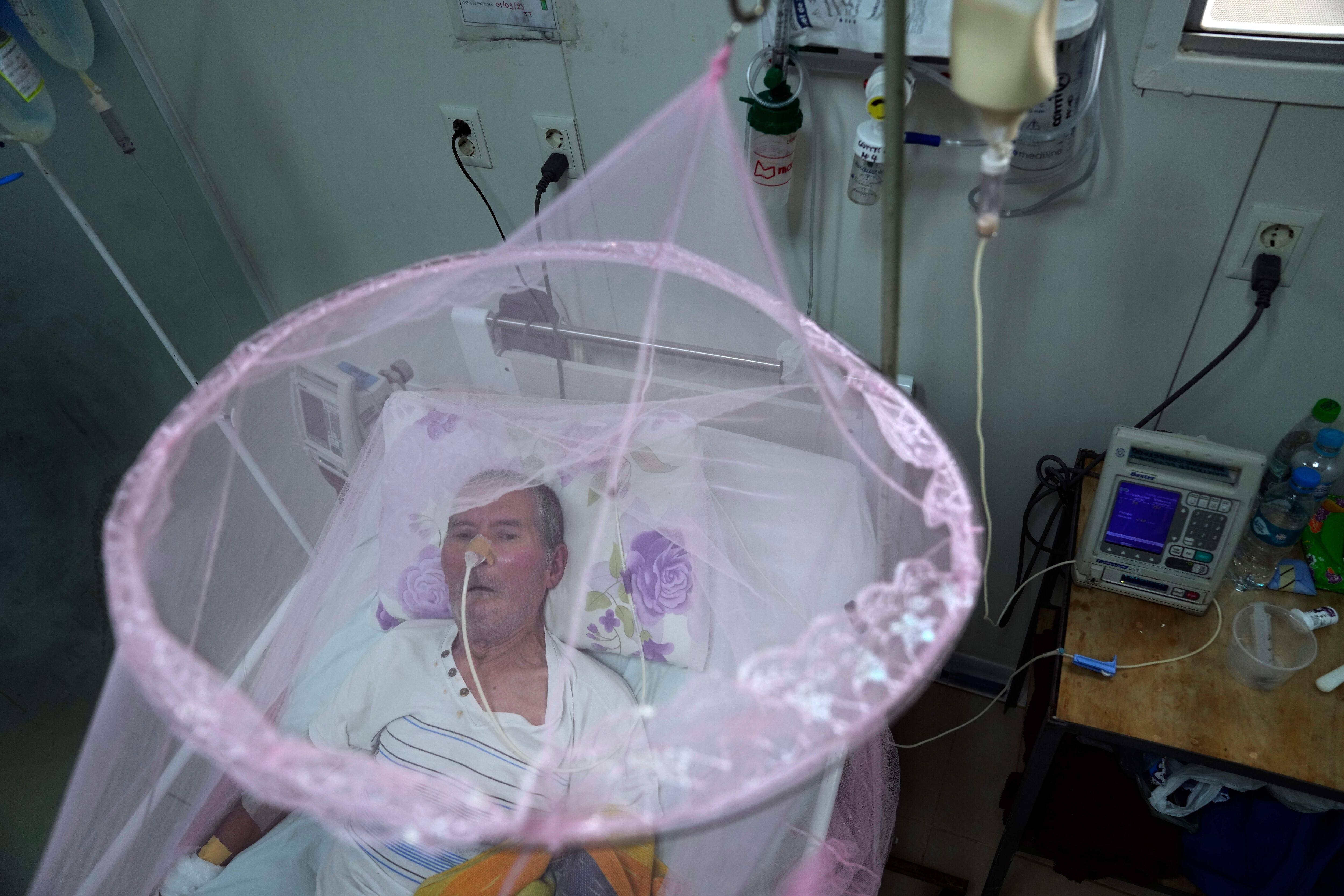
The European Medicines Agency (EMA) has recommended restricting the use of the first vaccine developed against chikungunya — an infectious disease transmitted by mosquitoes in tropical areas that causes, among other symptoms, severe joint pain that lasts for several months or even years — in people aged over 65. The measure comes after at least 17 serious adverse events, two of them fatal, were reported in people over 62 years of age who had received the single dose of Ixchiq, the drug’s brand name.
This vaccine uses a weakened version of the chikungunya virus, genetically modified to produce the body’s immune response without causing the disease. Although the investigation is ongoing and a causal relationship between the vaccination and the events has not yet been confirmed, the EMA highlights in a document dated March 2 that “the vaccine strain of the chikungunya virus was detected in the bodily fluids” of the two deceased individuals.
They are an 84-year-old man who developed encephalitis — a rare complication of chikungunya — and a 77-year-old man with Parkinson’s disease. The EMA, which approved the vaccine a year ago, notes in its initial communications that these safety concerns may be related to the underrepresentation of people over 65 in clinical trials.
“As the studies primarily included people under 65, and the vast majority of serious cases have been in people of that age or older, the EMA’s Pharmacovigilance Committee temporarily recommends restricting the use of the vaccine” in this latter group, as it is not considered safe for now.
“The European Commission has requested that the EMA issue an opinion on the matter by September 30, 2025,” a spokesperson for the agency responded in writing to EL PAÍS. He noted that while the research is ongoing, the use of the vaccine in people 65 and older is “contraindicated.” In the United States, the FDA has decided to adopt the same measure, but has lowered the age limit to 60.
The EMA spokesperson reports that “the Committee for Medicinal Products for Human Use (CHMP) was of the opinion [following the trial results and prior to the drug’s approval] that the population included in the safety studies was well distributed (with 346 participants aged 65 and over vaccinated out of a total of 4,643 adults) and no major safety concerns were identified.”
The percentage of those over 65 was 7.5%, a much lower proportion than the approximately 20% this age group represents in the EU population. The vaccine, produced by Valneva — which had not responded to questions from EL PAÍS by press time—is currently available in France, Germany, Sweden, the Netherlands, and Belgium, among other countries, as well as the United States and Canada, although not yet in Spain.
In recent months, the drug has begun to be used in areas with chikungunya outbreaks and among travelers visiting them. One of these is the French island of Réunion in the Indian Ocean, where authorities launched a vaccination campaign last January, specifically targeting those over 65. In total, according to provisional EMA data from late April, more than 40,000 people have received the dose worldwide: 19,000 of them in the United States, 12,000 in France — more than half in Réunion — and 6,000 in Canada.
Rosa López Gigosos, a member of the Spanish Association of Vaccinology (AEV), believes that the detected cases may constitute “an adverse effect of the vaccine not observed in the trials.” According to this expert, live attenuated virus vaccines generally produce more adverse reactions than those that use fractions of the pathogens, such as some of their proteins.
“These reactions are usually minor, like a mild fever, and short-lived. The yellow fever vaccine, for example, is also an attenuated virus and has been used since 1930 with very good results, although its use is also restricted in those over 60,” she adds.
In any case, López Gigosos continues, “in older people and those with underlying pathologies, it is generally more convenient to use other types of vaccines, such as protein vaccines, although these tend to produce less potent or shorter-lasting immune responses.”
Three months ago, the EMA approved a second protein-based chikungunya vaccine. Its name is Vimkunya and it was developed by the Danish pharmaceutical company Bavarian Nordic.
The limited participation of certain groups — women, the elderly, certain ethnic groups, patients with certain conditions — in clinical trials has been a recurring issue debated in medicine for decades. “It’s a very important issue because it can’t be guaranteed that a drug will have the same safety and efficacy results observed in trials if it’s then used in a population group not included or underrepresented in them,” explains Alberto Borobia, head of the Clinical Research and Clinical Trials Unit (UICEC) at La Paz Hospital (Madrid).
Borobia emphasizes that this isn’t the only prejudice underrepresented groups suffer in clinical trials. “For some pathologies, such as cancer, clinical trials are the last therapeutic step patients can take when they have exhausted all approved options. Here we are talking about an underserved population, because they don’t have access to trials due to certain conditions. A clear example in Spain is the population living in rural areas.”
La Paz Hospital is leading a European project called READI (Research in Europe and Inclusion of Diversity). This project, comprised of 73 organizations from 18 countries, aims to “improve the inclusion of underrepresented groups in clinical trials, such as the elderly, ethnic minorities, residents of rural areas, the blind, or citizens with low sociocultural backgrounds,” according to documentation published by the Madrid regional government.
Women are another group historically underrepresented in trials, even in diseases like Alzheimer’s, where they have a higher incidence. This was recently revealed by a joint study by the Barcelonaβeta Brain Research Center (BBRC), a research center of the Pasqual Maragall Foundation, and the Women’s Brain Foundation.
“The question we asked ourselves was: Why is there a lower presence of women?” says Anna Brugulat, a neuropsychologist at the Pasqual Maragall Foundation, postdoctoral researcher, and leader of the study. The answer, Borobia agrees, is multifactorial and influenced by both historical inertia — the presence of men has traditionally been dominant in trials — and structural factors.
“Some are associated with gender, such as family responsibilities, which lead some women not to participate due to lack of time and support. Others are due to the characteristics of the trial. Since the Alzheimer’s trials require a lumbar puncture, some women refuse it because they associate it with negative experiences with epidurals during childbirth,” explains this expert.
Miquel Serra, a researcher at the University of Zurich (Switzerland) specializing in Health Economics, and his team have extensively investigated the problem caused by the use of cancer drugs in patient profiles not included in clinical trials. “The root cause is often the design of the trials themselves, which is something pharmaceutical companies do and can sometimes influence the results by selecting patients who are more likely to achieve good results more quickly,” he explains.
In the case of cancer, he adds, this may involve “very strict inclusion criteria, applicable only to younger patients without other pathologies, who will be better able to withstand the toxicity of certain oncological therapies, which in turn will allow for better indicators that will favor their approval.”
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
[ad_2]
Source link






Comentarios